Catalyst For Fischer Esterification . This is known as fischer esterification. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as the catalyst. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst. This generates a highly activated form of the carbonyl. Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. An acid catalyst is required and the alcohol is. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol.
from www.masterorganicchemistry.com
An acid catalyst is required and the alcohol is. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as the catalyst. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. This generates a highly activated form of the carbonyl. Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. This is known as fischer esterification. First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst.
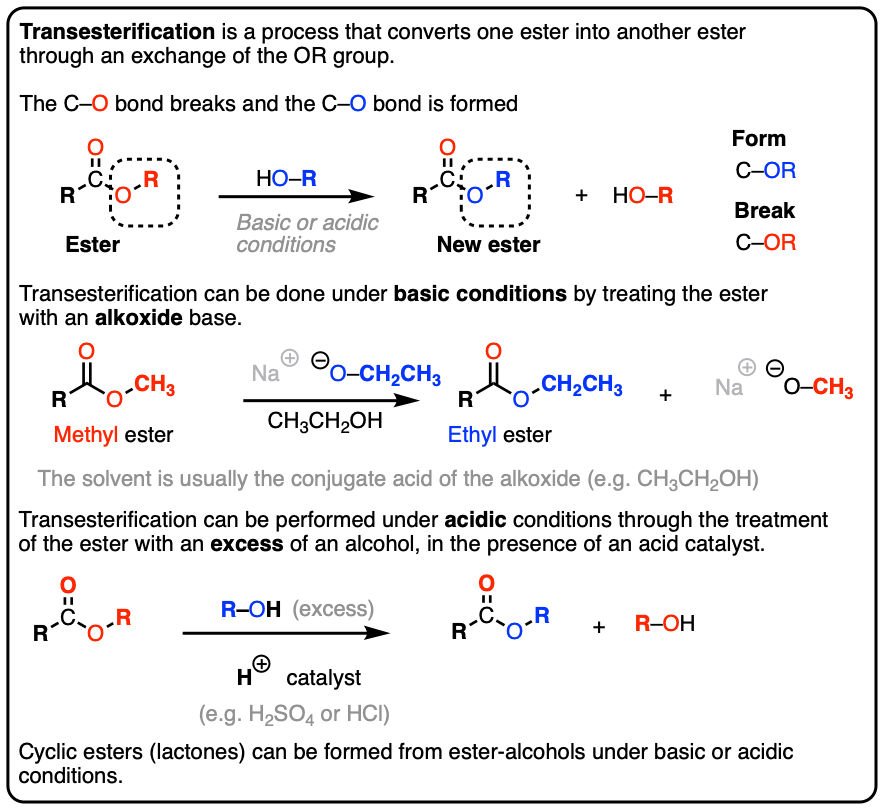
Transesterification Master Organic Chemistry
Catalyst For Fischer Esterification This generates a highly activated form of the carbonyl. First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst. This generates a highly activated form of the carbonyl. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. This is known as fischer esterification. An acid catalyst is required and the alcohol is. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as the catalyst. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol.
From ar.inspiredpencil.com
Fischer Esterification Mechanism Carboxylic Acid Catalyst For Fischer Esterification This is known as fischer esterification. First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst. Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. This generates a highly activated form of the carbonyl. An acid. Catalyst For Fischer Esterification.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols Catalyst For Fischer Esterification An acid catalyst is required and the alcohol is. First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. A wide range of aliphatic. Catalyst For Fischer Esterification.
From www.masterorganicchemistry.com
Acid Catalysis of Organic Reactions Why It Works Catalyst For Fischer Esterification This generates a highly activated form of the carbonyl. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. This is known as fischer. Catalyst For Fischer Esterification.
From www.pdfprof.com
esterification real life examples Catalyst For Fischer Esterification This generates a highly activated form of the carbonyl. An acid catalyst is required and the alcohol is. Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. Carboxylic acids can be converted to esters with an acid catalyst and. Catalyst For Fischer Esterification.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Catalyst For Fischer Esterification Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. This is known as fischer esterification. This generates a highly activated form of the carbonyl. An acid catalyst is required and the alcohol is. Fischer esterification is the esterification of a. Catalyst For Fischer Esterification.
From webapi.bu.edu
💌 Fischer esterification mechanism. Fischer Esterification Definition Catalyst For Fischer Esterification A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. This generates a highly activated form of the carbonyl. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of. Catalyst For Fischer Esterification.
From www.youtube.com
Fischer Esterification YouTube Catalyst For Fischer Esterification First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst. An acid catalyst is required and the alcohol is. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as the catalyst. Carboxylic acids can react with alcohols to form esters in a process called. Catalyst For Fischer Esterification.
From www.youtube.com
Fischer Esterification (Simplified) General Reaction YouTube Catalyst For Fischer Esterification A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. An acid catalyst is required and the alcohol is. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. This is known as fischer esterification. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction.. Catalyst For Fischer Esterification.
From www.coursehero.com
[Solved] Give the full mechanism for the process of an esterification Catalyst For Fischer Esterification Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst. An acid catalyst is required and the alcohol is. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong. Catalyst For Fischer Esterification.
From www.researchgate.net
The mechanism of Fischer esterification catalysed by Brønsted acidic Catalyst For Fischer Esterification Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as the catalyst. This is known as fischer esterification. This generates a highly activated form of the carbonyl. Carboxylic acids can. Catalyst For Fischer Esterification.
From www.youtube.com
Fischer esterification from App Chemistry" Basic Reaction Catalyst For Fischer Esterification Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol. Catalyst For Fischer Esterification.
From br.pinterest.com
Mechanism of AcidCatalyzed Hydrolysis of An Ester with a Tertiary Catalyst For Fischer Esterification Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. This generates a highly activated form of the carbonyl. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. Fischer esterification is the esterification. Catalyst For Fischer Esterification.
From www.mdpi.com
Catalysts Free FullText ZeoliteBased Catalysts A Valuable Catalyst For Fischer Esterification Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. First, the carbonyl oxygen of acetic acid is protonated by the acid catalyst. An acid catalyst is required and the alcohol is. This is known as fischer esterification. Typical fisher. Catalyst For Fischer Esterification.
From www.youtube.com
Esterification Reaction Organic Chemistry YouTube Catalyst For Fischer Esterification A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Carboxylic acids can be converted to esters with an acid catalyst and an excess of. Catalyst For Fischer Esterification.
From chem.libretexts.org
Fischer Esterification Chemistry LibreTexts Catalyst For Fischer Esterification A wide range of aliphatic and aromatic acids and alcohols were compatible and afforded the. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. This generates a highly activated form of the carbonyl. Carboxylic acids can be converted to esters with. Catalyst For Fischer Esterification.
From www.chegg.com
Solved 7) A Fischer esterification reaction is shown below Catalyst For Fischer Esterification Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. An acid catalyst is required and the alcohol is. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence. Catalyst For Fischer Esterification.
From ar.inspiredpencil.com
Fischer Esterification Mechanism Carboxylic Acid Catalyst For Fischer Esterification Carboxylic acids can react with alcohols to form esters in a process called fischer esterification. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. A wide range of aliphatic and aromatic acids and alcohols were compatible. Catalyst For Fischer Esterification.
From www.masterorganicchemistry.com
Transesterification Master Organic Chemistry Catalyst For Fischer Esterification This generates a highly activated form of the carbonyl. Graphene oxide is an efficient and reusable acid catalyst for the esterification reaction. Typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of corresponding. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. An acid catalyst is required. Catalyst For Fischer Esterification.